Clinical Study on Nonatypical Endometrial Hyperplasia (NAEH)
Nonatypical Endometrial Hyperplasia (NAEH)
The SUNFLOWER study is a Phase 3, open-label clinical research study on an investigational intrauterine system (IUS) in patients with a type of endometrial hyperplasia called nonatypical endometrial hyperplasia (NAEH). The purpose of this study is to learn how the investigational IUS works in the treatment of nonatypical endometrial hyperplasia.
Study information:
Condition: Contraception (Birth Control)
Location: Las Vegas, Nevada
Duration: Up to 10 months
Participation: Up to 5 visits
Compensation: Up to $1619
Eligibility:
Women age 18 or older
With known or suspected NAEH
May accept an intrauterine system or an oral progestin as a treatment for 6 months
Have not received progestin treatment for NAEH within the last 24 months
Other study requirements apply.
Participants may receive:
Compensation up to $1619
Regular check-ins with a team of doctors and nurses to monitor your health
All study-related care and medications will be provided at no cost
Travel expenses may be reimbursed
Get started today.
You are not committed to participate in a study by completing this form. Participation is voluntary, and you may leave at any time if you change your mind.
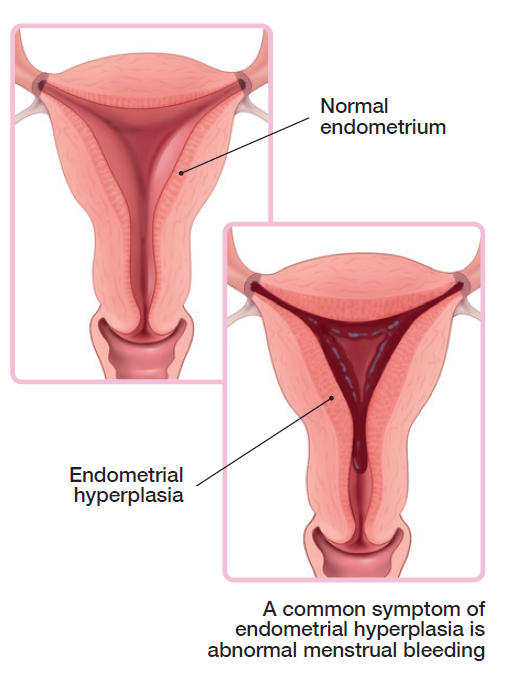
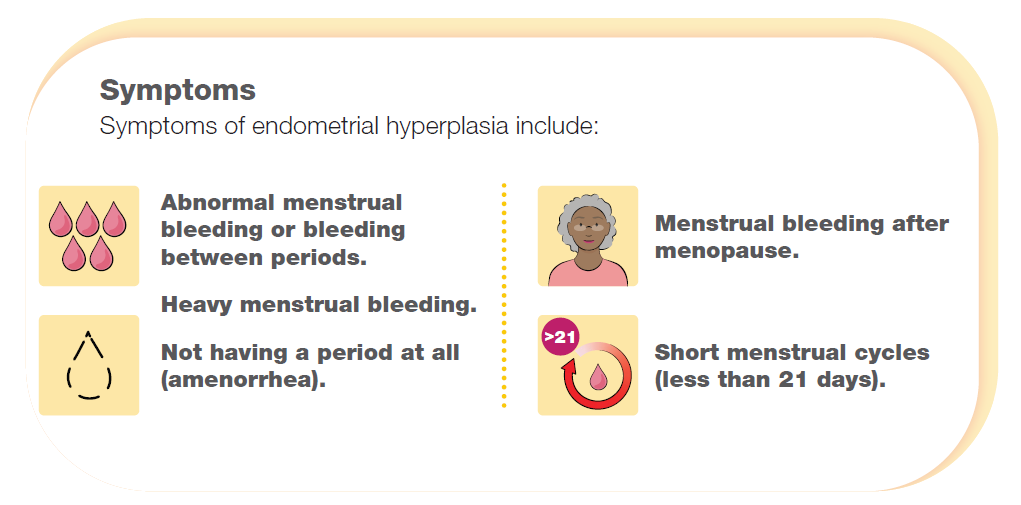
What is endometrial hyperplasia?
Endometrial hyperplasia happens when the inner lining of the uterus/womb, called the endometrium, grows excessively and becomes thicker. It is caused by too much estrogen, a hormone that is produced mainly by the ovaries. Endometrial hyperplasia is divided into 2 types:
Atypical endometrial hyperplasia (AEH), when the cells in the endometrium don't look normal. This type of hyperplasia can develop into endometrial cancer if left untreated.
Nonatypical endometrial hyperplasia (NAEH), when the cells in the endometrium look normal. This type of hyperplasia can also develop into endometrial cancer, but the risk is lower.
The purpose of this study is to learn how the investigational IUS works in the treatment of nonatypical endometrial hyperplasia. The study will also look at how an investigational oral progesterone tablet works in nonatypical endometrial hyperplasia.
The SUNFLOWER study is sponsored by Bayer and all information is provided by Bayer.