
Active Clinical Trials
Qualifying participants may receive compensation for time and travel and study-related healthcare at no cost.
Available Studies
-
Contraceptive Patch
Find out more about a clinical research study looking at a potential new progesterone-only contraceptive patch option for women to prevent pregnancy in healthy, sexually active women.

-
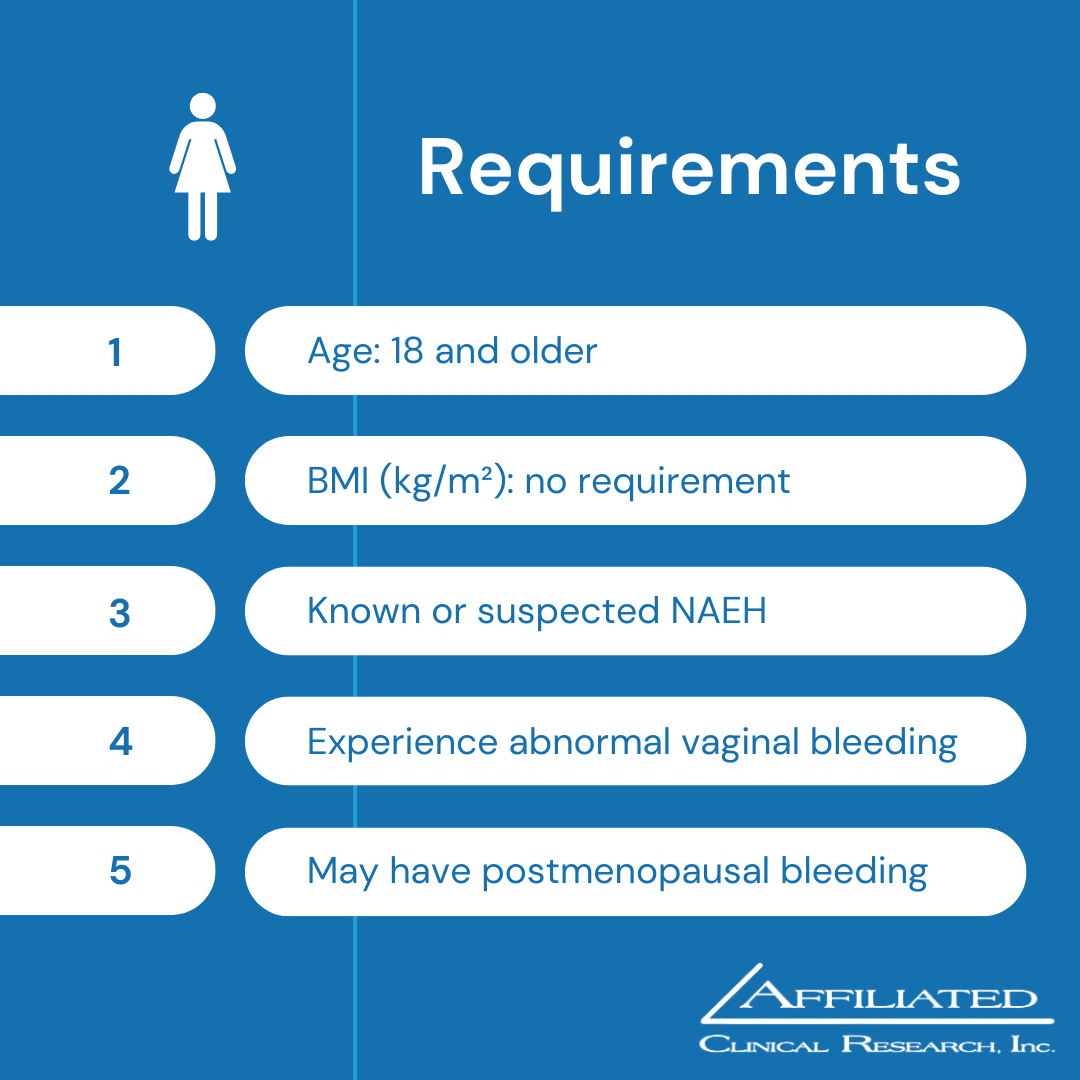
Nonatypical Endometrial Hyperplasia (NAEH)
Do you have known or suspected NAEH? Signs and symptoms of NAEH include abnormal vaginal bleeding including heavy, prolonged, sporadic, irregular, or postmenopausal bleeding.
-
Knee Osteoarthritis (OA)
Find out more about the experimental study medicine, pentosan polysulfate sodium (PPS), for the treatment of knee osteoarthritis (OA) pain given as an injection.

-
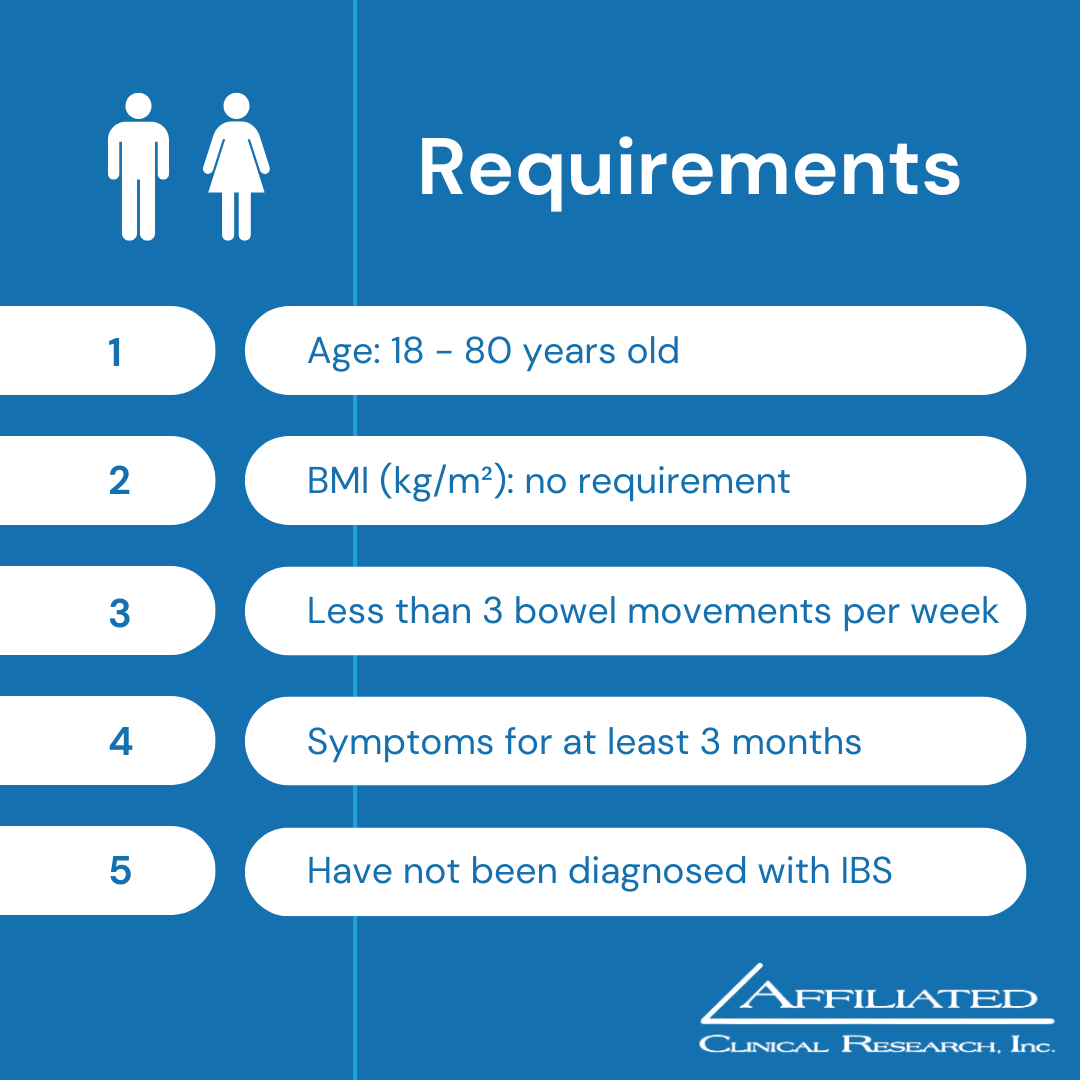
Chronic Idiopathic Constipation (CIC)
The ACCEL clinical research study is for adults who are living with chronic idiopathic constipation (CIC), also called functional constipation (FC).
-
Ulcerative Colitis (UC)
Have you been diagnosed with moderately to severely active Ulcerative colitis (UC) for at least 3 months? Learn more about an investigational treatment option.

-
Crohn's Disease (CD)
Have you been diagnosed with moderately to severely active Crohn’s disease (CD) for at least 3 months? Learn more about an investigational treatment option.

-
Upcoming Studies
Please see below for our upcoming clinical trials. Contact us through our inquiry form or directly call/text us at (702) 290-2950 for more information.


